Acquisition of IATA’s CEIV Pharma Certification : Validation of Incheon International Airport’s Capabilities in Transporting COVID-19 Diagnostic Kits and Vaccines
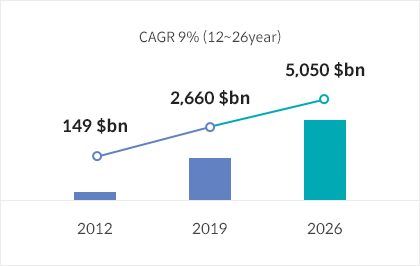
According to the UK-based market analysis firm, Evaluate Pharma, the global biopharmaceutical market is on a trajectory of consistent growth, with projections placing its worth at $505 billion by 2026. Recognizing this potential, Incheon International Airport is positioning itself at the forefront of the air transport market for pharmaceuticals. The airport’s efforts are concentrated on shaping an industrial ecosystem where all air transportation stakeholders can prosper collaboratively.
In 2019, Incheon International Airport, as a community entity, was bestowed the CEIV Pharma Certification from the International Air Transport Association (IATA). This recognition underscored the airport’s proficiency in handling temperature-sensitive pharmaceuticals such as vaccines. Following the outbreak of COVID-19, Incheon International Airport has showcased its unparalleled expertise in facilitating the global logistics of COVID-19 diagnostic kits and vaccines. Furthermore, in September 2022, the airport successfully secured recertification, underscoring its commitment to maintaining the highest standards in aviation transport quality. This recertification process, rigorous in nature, involves on-site audits and evaluations conducted every three years.
The CEIV Pharma Certification is a specialized quality assurance system from the International Air Transport Association (IATA) for the air transportation of pharmaceuticals, including vaccines.

Analysis of Biopharmaceutical Market Size